AUROBINDO PHARMA Limited – Committed to healthier life.
Date Published: September 18, 2023

Company Overview:
Founded in 1986 by Mr. P.V. Ramprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born of a vision. The company commenced operations in 1988-89 with a single unit manufacturing Semi-Synthetic Penicillin (SSP) at Pondicherry. Aurobindo Pharma became a public company in 1992 and listed its shares on the Indian stock exchanges in 1995. In addition to being the market leader in Semi-Synthetic Penicillin’s, Aurobindo Pharma has a presence in key therapeutic segments such as neurosciences (CNS), cardiovascular (CVS), anti-retroviral, anti-diabetics, gastroenterology and Anti-biotics. Through cost effective manufacturing capabilities and a few loyal customers, the company also entered the high margin specialty generic formulations segment. Today Aurobindo Pharma has evolved into a knowledge driven company manufacturing active pharmaceutical ingredients and formulation products. It is R&D focused and has a multi-product portfolio with manufacturing facilities in several countries. The formulation business is systematically organized with a divisional structure, and has a focused team for key international markets. Leveraging its large manufacturing infrastructure for APIs and formulations, wide and diversified basket of products and confidence of its customers, Aurobindo achieved revenue of USD 2.6 billion in FY2017-18. Aurobindo’s 11 units for APIs / intermediates and 15 units (10 in India, 3 in USA, 1 in Brazil and 1 in Portugal) for formulations are designed to meet the requirements of both advanced as well as emerging market opportunities.
Aurobido is well-integrated pharma company, it features among the top 2 Pharmaceutical companies in India in terms of consolidated revenues. Aurobindo exports to over 150 countries across the globe with around 90% of revenues derived from international operations. Our customers include premium multi-national companies. With multiple facilities approved by leading regulatory agencies such as USFDA, EU GMP, UK MHRA, South Africa-MCC, Health Canada, WHO and Brazil ANVISA, Aurobindo makes use of in-house R&D for rapid filing of patents, Drug Master Files (DMFs), Abbreviated New Drug Applications (ANDAs) and formulation dossiers across the world. Aurobindo Pharma is among the largest filers of DMFs and ANDAs in India. Company has One of the largest R&D facilities in India, Aurobindo Pharma has five research centres spread over 16,000 square meters. It also has 2 R&D centers in USA. The company employs over 1560 scientists & analysts in- house expertise in product development ensuring a quick turnaround time in areas such as: Project / product identification, Literature evaluation / patent study, API process development, Formulation development, Pilot BA / BE, Exhibiting batches for dossier submission, Stability studies for global requirements, Dossier submission
Industry Overview
The Indian pharma, which has a strong footprint in the generics segment grew by nearly 5 per cent on a y-o-y basis to $49.78 billion in FY23, and is expected to reach $57 billion by FY25, according to the CareEdge report. During FY18–FY23, the sector, including domestic and exports, registered a compound annual growth rate (CAGR) of 6–8 per cent with 8 per cent growth in exports and 6 per cent growth in the domestic market during the same period. Compared to FY22, exports grew by just 3 per cent while the domestic market continued to grow at a healthy rate of 7 per cent in FY23.
Indian pharmaceutical companies are well placed to futile the opportunities, as they are competitive globally and hold a sizeable market share in most markets. Moreover, other factors such as 1) long‐term opportunities in the U.S. with increasing preference for specialty/complex generics (including biosimilars) and injectables, 2) expected healthy growth in IPM, which is expected to stage high single to double‐digit growth in FY2024 as well, and 3) emerging opportunities in the API space would be key growth drivers over the long term; while in the near to medium term, cost pressures could act as headwinds. Collectively, this points towards a strong growth potential over the long term for Indian pharma companies. Generics and specialty product manufacturers outperformed led by strong growth in the US that was driven by volume growth in the base business for both generic and specialty products and new launches. It was also aided by strong growth in other advanced and emerging markets, including in India. Regularization and improvement in demand, post COVID -19 phase, ac ted as the driver among outperforming API players. Vaccine production will continue to support total pharmaceuticals production over the next several years, albeit to a lesser extent than during 2021. Pharmaceutical companies are developing booster shots which target new variants of the coronavirus, some of which major health authorities have now approved for administration. Emerging markets will gradually gain a larger share of global production, as improvements to healthcare systems increase accessibility. The developed world will remain an important area for pharmaceuticals as its ageing populations create demand for high value-added specialty products aimed at chronic diseases. Over the longer term, we believe India could benefit from global investors’ partial shift away from China amid geopolitical uncertainty. This will further bolster India’s path to becoming one of the world’s largest pharmaceuticals manufacturers over the next decade which is supported by demographic factors and its large generic sector.
TECHNICAL VIEW:
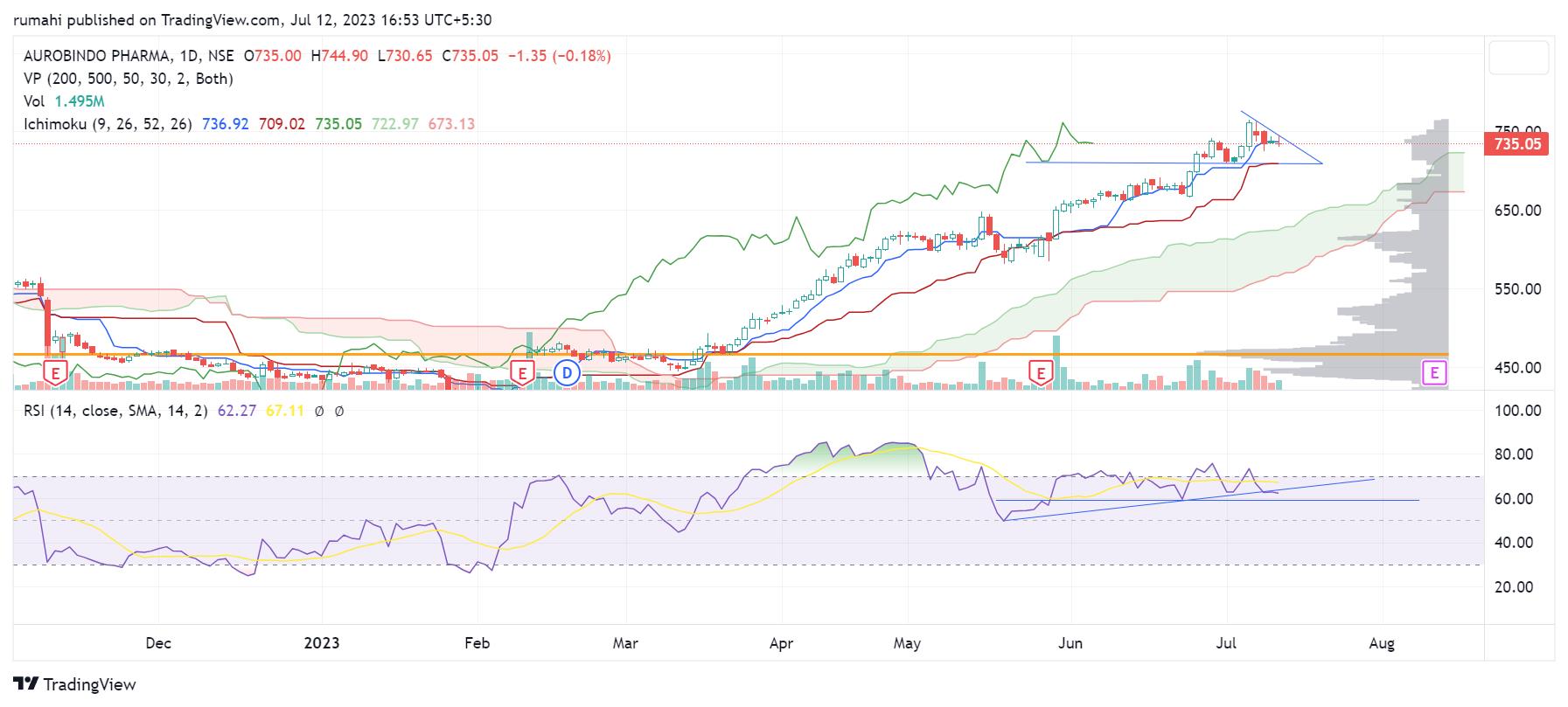
Technically Aurobindo pharma making higher high higher low on daily chart since 27th march 2023 from levels of 511 when stock closed above 500. And it continued to outperform Pharma index after long time with strong volume and price breakout on daily chart now stock is making Bullish Flag pattern with strong support near levels of Rs 700, We suggest to Buy Aurobindo at price recommended near 719 with strong support near levels of 670 for target price of 860, followed by next target of 930 for a period of 1 year.
Revenue improved in US and API businesses:
Aurobindo Pharma had a satisfactory performance in the current quarter due to stable demand and pricing conditions across their portfolio and geographical regions. In Q4FY23, the company's revenue reached INR 64,730 mn, showing an 11.4% increase compared to the previous year and a 1.0% increase compared to the previous quarter. This growth was primarily driven by the US market, growth markets, and the API segment. The US revenue accounted for 47.0% of the total revenue, with a YoY increase of 11.6% to INR 30,450 mn and a QoQ increase of 1.5%. The US injectable business revenue saw a 3% YoY increase and an 18% QoQ increase, amounting to USD 71.9 mn. The combined sales of specialty OSD and other Eugia specialties were USD 81 mn during the quarter. Europe revenue increased by 7.7% YoY to INR 16,600 mn, representing 25.6% of consolidated revenues. In Euro terms, Europe revenue grew by 3.0% YoY to EUR 188 mn. The growth markets saw a significant YoY growth of 51.3% and a QoQ growth of 18.7%, contributing 5.2% to the revenue. The ARV business revenue for Q4FY23 was INR 1,590 mn, accounting for 2.5% of total revenue. The API business contributed 15.7% to the revenue, generating INR 10,170 mn, with a YoY growth of 11.4% and a QoQ growth of 6.5% driven by increased demand for key products. Aurobindo is well-positioned to capitalize on favorable market conditions, including a rising number of drugs in short supply, reduced competition, and a consistent pace of approvals for the company. Furthermore, the inclusion of g-revlimid starting from the Q3FY24 is expected to support the growth of Aurobindo Pharma's US business.
KEY CON-CALL HIGHLIGHTS
- Margin between 15.5-20% on a quarterly basis and ex-Revlimid should be between 15% and 20%
- US generic injectable business growth in double digit in FY24 and FY25; ex-injectable growth in higher single digit
- Bulk of volumes awarded to be seen in Q1 FY24 onwards in US; still a fair amount of market uncovered in terms of ANDAs
- Prices have stabilized in US and in a better position compared to what we saw in first 9 months and that trend continues in April and May
- Eugia - US$411mn of sales in FY24 which is flat YoY and based on current pipeline, should continue with double digit growth + Revlimid being additional – Gross margin of 69-70% and margin of 25-30%
- Acrotech – maintaining at US$25-30mn per quarter run rate and ramping up could take time
- PLI capex to be completed by Mar’24; expect good cash generation from FY25 as all capex stabilizes
- Vizag plant – started exhibit batches and first filing in Sep’23 and for European and EM markets. Can be used for US too and on track
- R&D – Incurring Rs4bn per quarter to be the trend
- About Rs450mn in one-offs in revenue and costs in equal proportion in Q4
- Biosimilars to contribute from FY25
- First biosimilar filing in India in July, EU in Sep and US by Q4; do not do any interchangeability trial as believe such a concept may go away few years down the line
- Got incentives of Rs480mn for domestic capex and incentives have picked up a lot
- Focusing on onco and anti-diabetes peptides and filed DMF for Liraglutide and another GLP 1 in this year
- Maintenance capex US$125-130mn.
On track current investments:
Pen-G project is on fast track with US$130-140mn already spent with total purchase order issued till date amounting to US$200mn. While, overall investment is expected to be US$250mn. The project has a market size of US$250mn and is expected to add business FY25 onwards. On the Biosimilar front, The R&D spend has been higher for the quarter due to ramp-up in spending for clinical trials related to biosimilar. The trend is expected to continue for few more quarters due to ongoing phase-III trials for 3 products and having 30-40 patient recruitments.
Valuation and outlook:
While the management remain optimistic on its outlook and maintained guidance of 20% margin in coming years (17%-18% margin in FY24). Further, the management indicated about the expansion plans, 3 plants being expanded, and of which 1 is Eugia, to be commercialized by FY24/25. China OSD facility, which will firstly start manufacturing for EU market in Q1FY25 and then later for China by Q4FY25. Biosimilar plant expected to start in CY23 end, and lastly PLI capex, to be completed by end FY24. It expects sales during Q1/ Q2FY24 to increase led by new launches, Q3FY24 sales growth led by the launch of Revlimid, and a biosimilar launch by Q4FY24. Maintain BUY and add for price target of 860-930 for period of 1-year considering forward EPS of 44.76 and 51.95 for F.Y 2023-24 and F.Y 2024-25.