Pharmaceuticals Sector
Date Published: February 16, 2021

The Pharma & Healthcare industry comprises the manufacture of pharmaceuticals for human and veterinary use, medical equipment and related supplies. It also includes providers of ambulatory health services, medical and diagnostic laboratories, hospitals, and nursing and residential care facilities. The need for medication for treatment of various diseases continues to be key driver for pharmaceuticals products. Medicine spending in India is projected to grow 9 12% over the next five years, leading India to become one of the top 10 countries in terms of medicine spending.India plans to set up a nearly Rs 1 lakh crore (US$ 1.3 billion) fund to provide boost to companies to manufacture pharmaceutical ingredients domestically by 2023.
Going forward, better growth in domestic sales would also depend on the ability of companies to align their product portfolio towards chronic therapies for diseases such as such as cardiovascular, anti-diabetes, anti-depressants and anti-cancers, which are on the rise.
The pharmaceutical industry comprises of discovery, development, manufacturing and marketing of finished pharmaceuticals products (made from medicinal substances) for human and veterinary use. The need for medication for treatment of various diseases continues to be key driver for pharmaceuticals products. Additionally, the sector is characterized by investment in research and development for continual innovation of better products both in terms of cost and effectiveness.
PHARMACEUTICAL INDUSTRY VALUE CHAIN
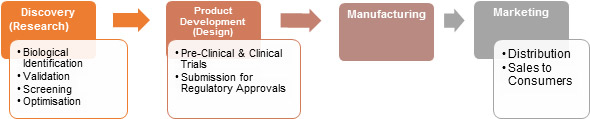
Discovery (Research)
The process involves the identification of candidates, synthesis, characterization, screening, and assays for therapeutic efficacy. Once a compound has shown its value in these tests, it will begin the process of drug development prior to clinical trials.
Pre-clinical Trials
After a compound has been identified as promising, it enters a rigorous testing and evaluation stage, known as Preclinical. This stage is designed to assess the chemical properties of the new drug as well as to determine the steps for synthesis and purification. In this stage, the toxicity and pharmacological effects of the drug are evaluated through animal testing. These studies are both short and long-term. The information collected from these studies is vital so that safe human testing can begin.
Regulatory Approval before clinical trial (IND application)
An Investigational New Drug (IND) Application is filed with the Food and Drug Administration prior to human testing. The IND application is a compilation of all known information about the compound,including how it is manufactured. It also includes a description of the clinical research plan for the product and the specific protocol for phase 1 human clinical trials. Unless the FDA specifically objects, the IND is automatically allowed after 30 days and clinical trials can begin.
Clinical Trials
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety (or more specifically, information about adverse drug reactions and adverse effects of other treatments) and efficacy data to be collected for health interventions (e.g., drugs, diagnostics, devices, therapy protocols). These trials can take place only after satisfactory information has been gathered on the quality of the non-clinical safety, and Health Authority/Ethics Committee approval is granted in the country where the trial is taking place.Depending on the type of product and the stage of its development, investigators enroll healthy volunteers and/or patients into small pilot studies initially, followed by larger scale studies in patients that often compare the new product with the currently prescribed treatment. As positive safety and efficacy data are gathered, the number of patients is typically increased. Clinical trials can vary in size from a single center in one country to multicenter trials in multiple countries.
Regulatory Approval after clinical trial (FDA application)
All information about the drug gathered during the drug discovery and development process is assembled in the New Drug Application (NDA). During the review period, the FDA may ask the company for additional information about the product or seek clarification of the data contained in the application.
Market Segmentation
The pharmaceutical industry may be classified in two ways, one by way of geography and another on the basis of products.
Geographical Classification
The global pharmaceuticals industry can be classified into two categories based on geography:
- Regulated and
- Semi-regulated (or emerging) markets.
The regulated markets include developed countries such as the United States, UK, Germany, France, Italy,Canada, Japan and Australia and are primarily governed by stringent government regulations such as intellectualproperty (IP) protection, product patent recognition etc. As a result, regulated markets have greater stabilityfor both volumes and prices when a drug is under patent protection. Whereas, semi-regulated markets includes developing countries (emerging markets of Asia, Africa,Latin America) such as Brazil, Russia, India and China, which have lower entry barriers in terms of regulatoryrequirements; hence they are highly competitive. This requires companies to promote their products and therefore industry players compete on thebasis of brand, marketing and promotion and price.
Product Classification
The global pharmaceuticals industry can be classified into two categories based on patent status of the products:
- Patented products and
- Generic products.
Companies which hold patents for their products are given the right to exclude others from usingtheir patented products for any commercial purpose. This provides protection to the innovator to make good the initial costs incurred by him, viz. research development and marketing costs. On the other hand, Generic products are thosewhich are comparable to patented productsbut are not protected by patents. Generic drugs are subject to the regulations of the governments of countries where they are dispensed. They are copies of brand-name drugs and are the same as those patent products in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use. These drugs are cheaper as compared to patented drugs as generic manufacturers do not incur the investment costs of the innovator of a new drug. As a result, these drugs are available at a much lower price post the expiry of the patent.

Introduction
The pharmaceutical industry comprises of discovery, development, manufacturing and marketing of finished pharmaceuticals products (made from medicinal substances) for human and veterinary use. The need for medication for treatment of various diseases continues to be key driver for pharmaceuticals products. Additionally, the sector is characterized by investment in research and development for continual innovation of better products both in terms of cost and effectiveness.
History of the Industry
The Indian pharmaceutical industry can be said to have begun with the setting up of ‘Bengal Chemical and Pharmaceutical Works’ in Calcutta. Post-independence, many other public sector companies such as Hindustan Antibiotics Ltd. and Indian Drugs and Pharmaceuticals Ltd. were set up to reduce the imports of important antibiotics and also to meet the county’s demand from indigenous production.The industry is conspicuous by the large presence of private sector which has captured a substantial share in the domestic & external market. The public sector as in many other sectors contributed to strategic areas but has gradually been overtaken by the private players.Indian owned firms currently account for 80% of the domestic market, up from less than 20% in 1970.
The Indian companies have geared up not only the domestic production capacity but have a presence in a large number of countries including USA and countries of Europe as well as expanded through acquisitions in these countries. Thus, the industry which was predominantly driven by multinational companies in the 1950’s moved towards indigenous manufacturing and imports in the 1970’s and then to the present status of significant indigenous production and subsequent exports.
The Indian companies have geared up not only the domestic production capacity but have a presence in a large number of countries including USA and countries of Europe as well as expanded through acquisitions in these countries. Thus, the industry which was predominantly driven by multinational companies in the 1950’s moved towards indigenous manufacturing and imports in the 1970’s and then to the present status of significant indigenous production and subsequent exports.
Over-the-counter products
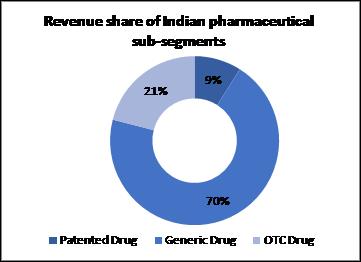
‘OTC Drugs’ means drugs legally allowed to be sold ‘Over the Counter’ by pharmacists, i.e. without the prescription of a Registered Medical Practitioner. Some of the examples of OTC products are Dettol, Band-Aid, Strepsils, Clearsil, energy-supplement Revisal, antacid Gelusil, pain balm and spray Volini etc. The OTC segment is one of the potential growth drivers for the Indian Pharma industry, as the sale of OTC drugs in India has been increasing over the years. As per a report by PwC, the OTC market is expected to grow at a CAGR of 18% and would be worth $11 billion in 2020 from a level of $1.8 billion in 2009. Although the phrase ‘OTC’ has no legalrecognition in India, all the drugs notincluded in the list of ‘prescription-onlydrugs’ are considered to be nonprescriptiondrugs (or OTC drugs).Some of the examples of OTC products are Dettol, Band-Aid, Strepsils, Clearsil, energy-supplement Revital, antacid Gelusil, pain balm and spray Volini etc. OTC drugs constitute 21 per cent of market share.
Key Drivers of the OTC Segment
- Enjoys wider distribution channel i.e. can be sold in department stores as well
- Public advertising of OTC products is allowed and thus they enjoy greater freedom to use more creative methods for marketing of OTC products.
- An increased consumer awareness on the basis of self-medication ofcommon ailments
Structure of Pharma sector in India:

Patented Drug
When a new drug product is invented by a pharmaceutical company, the innovating company gets exclusive rights to produce and sale that drug for some time. Such drugs are termed as ‘Patented’. In India patent for drugs is given under Indian Patent Act 1970. Generally the patent is given for a period of 20 years from date of filling application. Patented drugs constitute for 9 per cent of market share of India pharmaceutical sector.
Generic
Once the patent protection expires, other manufacturers are free to make copies of the innovator’s product and compete against innovator. A generic drug does not carry a brand name. These drugs are generally less expensive that the patented or branded drugs. Generic drug forms the largest segment of Indian pharmaceutical sector with 70 per cent of market share. India supplies 20 per cent of global generic medicines market exports, in terms of volume, making the country the largest provider of generic medicines globally and expected to expand even further in coming years.
Difference between branded generics and generics

In India, any non-patented moleculewith a brand name other than theinnovator’s name is termed as abranded generic. Some of the examples of branded generic in India is Calcium Sandoz, Otrivin Nasal drops, Benadryl etc. Chemically, brandedgenerics are identical, or bioequivalentto innovator drugs. Inthe global context, substitution – whenan innovator product goes off-patent -is the key driver for generics. Whereas, generics are drugsmade by or under license from theinnovator company and sold without abrand name.
Financial Performance of the Drugs and Pharmaceuticals Industry

Indian pharmaceutical sector is expected to grow to US$ 100 billion, while medical device market is expected to grow US$ 25 billion by 2025. Pharmaceuticals export from India stood at US$ 20.70 billion in FY20. Pharmaceutical export includes bulk drugs, intermediates, drug formulations, biologicals, Ayush and herbal products and surgical.India’s biotechnology industry comprising biopharmaceuticals, bio-services, bio-agriculture, bio-industry, and bioinformatics is expected grow at an average growth rate of around 30% a y-o-y to reach US$ 100 billion by 2025.This segment of Industry has shown tremendous progress in terms of infrastructure development, technology base and wide range of products. The industry has developed excellent GMP (Good Manufacturing Practices) compliant facilities for the production of different dosage forms. The strength of the industry is in developing cost effective technologies in the shortest possible time for drug intermediates and bulk activities without compromising on quality. This is realized through the country’s strengths in Organic Chemical Synthesis and Process Engineering. The domestic Pharma Industry has recently achieved some historic milestones through a leadership position and global presence as a world class cost effective generic drugs manufacturer for lifesaving drugs used for life threatening diseases for e.sg. AIDS, cancer etc.
Along with Brazil & China, India has carved a niche for itself by being a top generic Pharma player. Many Indian companies are co-partnering with foreign voluntary organizations for making available cheap generic drugs to lesser developed countries like Mozambique, Rwanda, South Africa and Tanzania which have about 33% of all people living with AIDS in Africa. USA is also sourcing anti-Retroviral drugs from Indian Companies which are approved by USFDA, under many schemes. This is because the Indian Pharmaceutical companies maintain highest standards in purity, stability and safety, health and environmental Protection.

Top Indian Pharma companies have got various international regulatory approvals for their plants, from agencies like USFDA, MHRA-UK, TGA-Australia, MCC-South Africa etc. Outside USA, India has the highest number of USFDA approved plants for generic drugs manufacture. Major share of Indian Pharma exports is sourced to developed western countries especially USA. It speaks not only about the excellent quality of Indian pharmaceuticals but also about the reasonableness of the prices. Some of the leading Indian Pharma companies derive about 50% of their turnover from International business.
The Pharma & Healthcare industry is of vital importance for human and economic development, providing a wide range of goods and services relevant to the promotion of health at all stages in the healthcare process – from prevention and treatment to rehabilitation and palliative care. It is one of the fastest-growing industries, supported by profound demographic changes, the rising incidence of non-communicable diseases, and surging per capita health spending in both developed and emerging markets. The industry is also one of the major innovators, with increasing expenditures on development of new molecules, biotechnology and adoption of new technologies that provide advanced healthcare andensure the longevity of the population.
Foreign Direct Investment in Pharmaceuticals Sector
India liberalized its FDI policies to allow up to a 100% FDI stake in ventures across the diverse sectors. It has substantially reduced industrial licensing requirements, removed restrictions on expansion and facilitated easy access to foreign technology and foreign direct investment FDI. Keeping in mind the increased importance of FDI and favorable changes in domestic system (legal and administrative), researcher in this research article has analyzed the development and status of FDI in pharma sector.A foreign investment could be a direct or portfolio investment. A direct investment is an acquisition or construction of physical capital by a firm from one (source) country in another (host) country. The FDI is an investment that involves a long- term relationship and control by a resident entity of one country, in a firm located in a country other than that of the investing firm. There is more involved in the direct investment than only money capital, for instance, managerial or technical guidance.
Driving Force behind FDI: Foreign Direct Investment has accelerated remarkably in the last decades and many of the major corporations of most developed countries have taken their production of goods to diverse parts of the world. Investments are most likely to take place where location and comparative advantages are present and FDI will presumably be concentrated to the regions where the industry in question has performed most efficiently. In order to compete in foreign markets, multinational companies take advantage of their firm- specific resources, such as technological and marketing expertise. The Union Cabinet has given its nod for the amendment of the existing Foreign Direct Investment (FDI) policy in the pharmaceutical sector in order to allow FDI up to 100 per cent under the automatic route for manufacturing of medical devices subject to certain conditions.The drugs and pharmaceuticals sector attracted cumulative FDI inflow worth US$ 16.50 billion between April 2000 and March 2020 according to the data released by Department for Promotion of Industry and Internal Trade (DPIIT).
Important segments in Indian Pharmaceutical Sector

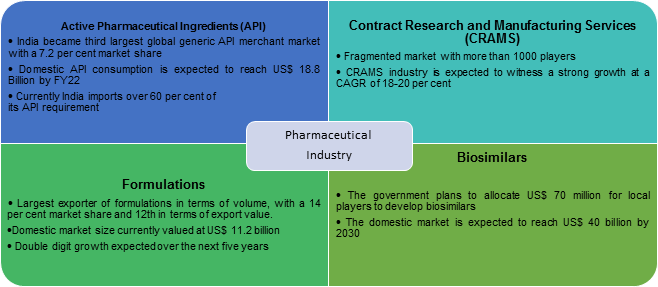
Formulations: Formulation is the process in which different chemical substances along with the API (Active pharmaceutical ingredients) are combined to produce the final product (medicine). The process also involves studying the compatibility of each substance involved in drug manufacturing and studying the impact/reaction of final drug.
Clinical Trials:Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety (or more specifically, information about adverse drug reactions and adverse effects of other treatments) and efficacy data to be collected for health interventions (e.g., drugs, diagnostics, devices, therapy protocols). These trials can take place only after satisfactory information has been gathered on the quality of the non-clinical safety, and Health Authority/Ethics Committee approval is granted in the country where the trial is taking place. Depending on the type of product and the stage of its development, investigators enroll healthy volunteers and/or patients into small pilot studies initially, followed by larger scale studies in patients that often compare the new product with the currently prescribed treatment. As positive safety and efficacy data are gathered, the number of patients is typically increased. Clinical trials can vary in size from a single center in one country to multicenter trials in multiple countries.
Regulatory Approval after clinical trial (FDA application): All information about the drug gathered during the drug discovery and development process is assembled in the New Drug Application (NDA). During the review period, the FDA may ask the company for additional information about the product or seek clarification of the data contained in the application.
API Active Pharmaceutical Ingredients: Active Pharmaceutical Ingredient is a term used to refer to the biologically active component of a drug (e.g. tablet, capsule). There are various chemicals included in the drugs and the portion that actually works in treating the condition i.e. the active part is known as ‘Active Pharmaceutical Ingredients’ or API in short. Sometimes a drug may also contain several API and the reaction or response to any particular drug will depend on the dosage prescribed, which varies from person to person. The function of the API is not limited to treatment or cure of the disease or symptoms alone though, they are also used for diagnosis or prevention at times.While many pharmaceutical companies are located in the United States and England, most API manufacturers are overseas. The largest are located in Asia, particularly in India and China. More and more companies are turning to outsourcing to API manufacturers like Dr. Reddy’s to cut costs on expensive equipment, employees, and infrastructure.
CRAMS: CRAMS is defined as the process of outsourcing research services/ product manufacturing activities to organizations which can provide the service at a low cost. CRAMS basically consists of the following two activities: contract research and contract manufacturing. CRAMS is mainly used in pharmaceutical sector because it requires extensive R&D and large scale manufacturing facilities. India offers significant cost advantage over mature manufacturing hubs in Europe and North America.
Biosimilars: A biosimilar is exactly what its name implies — it is a biologic that is “similar” to another biologic drug already approved by the FDA. When the patent surrounding a biologic’s formula is no longer protected, multiple companies can release a drug with the same chemical recipe, driving the cost down. That new biologic drug which is almost an identical copy of an original product is a biosimilar.
Exports
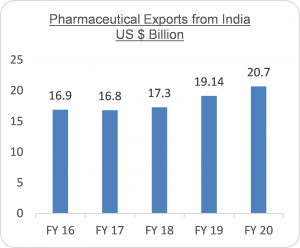
The Indian pharmaceuticals market is the third largest in terms of volume and the 13th largest in terms of value. It has established itself as a global manufacturing and a research hub. A large raw material base and the availability of skilled workforce gives the industry a definite competitive advantage. The Indian pharmaceutical industry is expected to grow at a compound annual growth rate (CAGR) of 22.4%. Indian drugs are exported to more than 200 countries in the world, with the US as the key market. Generic drugs account for 20 per cent of global exports in terms of volume, making the country the largest provider of generic medicines globally and expected to expand even further in coming years. Pharmaceutical exports from India, which include bulk drugs, intermediates, drug formulations, biologicals, Ayush & herbal products and surgicals reached US$ 19.14 billion in FY19 and US$ 20.7 billion in FY20.
Medical devices industry in India has been growing 15.2 per cent annually and expected to grow and reach US$ 25 billion by 2025. Presently there are 10,500 manufacturing units and over 3,000 pharma companies in India, growing at an exceptional rate. Globally more than 90 per cent of formulations approvals for Anti-retroviral (ARVs), Anti-tubercular & Anti-malarial (WHO pre-qualified) has been granted to India. Manufacturing costs in India are approximately 35-40 per cent of those in the US due to low installation and manufacturing costs.
Research & Development Spending by Pharmaceutical Companies:
On average, pharmaceutical companies spend 17% of revenues on research and development (R&D). Pharmaceutical companies are heavily reliant on research and development as their success is contingent on the development of new drugs.The pharmaceutical industry’s lifeblood is R&D. The success of major drug companies is almost wholly dependent upon the discovery and development of new medicines, and their allocation of capital expenditures reflects that fact. Although the average spending is 17% of revenues, some companies spend substantially more.As of 2020, many of the largest pharmaceutical firms spend nearly 20% on R&D. Of the 20 largest R&D spending industries in the world, the pharmaceutical industry makes up nearly half the list.
Key drivers of growth:
- Accelerated growth in Indiadriven by increased accessibility and affordability.
- Potential breakthroughs in next-generation innovative products.
- Strong growth in the US market by driving higher ANDA share in molecules going off patent, and potential ease in price erosion.
- Increased growth in large underpenetrated markets s such as Japan and China.
Government Initiatives:
The Indian government has taken many steps to reduce costs and bring down healthcare expenses. Speedy introduction of generic drugs into the market has remained in focus and is expected to benefit the Indian pharmaceutical companies. In addition, the thrust on rural health programs, lifesaving drugs and preventive vaccines also augurs well for the pharmaceutical companies.
Pharma Vision 2020
The Department of Pharmaceuticals has drafted Pharma Vision 2020 document, with an aim to establish India as a leading county for end-to-end drug manufacturing and innovation. This initiative by the government aims at providing support to Indian pharmaceutical sector through state of- the-art infrastructure,internationally competitive scientific research personnel for pharmaceutical R&D, and funding for research in the public and private sectors.
Reduction in approval time for new facilities
The time limit given for submitting the application for grant has been reduced to 4 months from 12 months, providing an extension of 2 months under the Draft Patents (Amendment) Rules, 2015. In order to compete with global players in pharmaceutical industries, approval process of drugs have been simplified by the authorities and approval time for new facilities has been drastically reduced. Also NOC for export license issued in 2 weeks compared to 12 weeks earlier.
Bulk Drug Parks
The Government of India plans to incentivize bulk drug manufacturers, including both state-run and private companies, to encourage ‘Make in India’ programme and reduce dependence on imports of active pharmaceutical ingredients (API), nearly 85 per cent of which come from China. Telangana has proposed to set up India’s largest integrated pharmaceutical city spread over 11,000 acres near Hyderabad, complete with effluent treatment plants and a township for employees, in a bid to attract investment of Rs 30,000 crore (US$ 4.5 billion) in phases.
Research and Development
The Department of Pharmaceuticals has planned to launch a venture capital fund of Rs 1,000 crore (US$ 154 million) to support start-ups in the research and development in the pharmaceutical and biotech industry.
National Health Protection Scheme
The National Health Protection Scheme is largest government funded healthcare programme in the world, which is expected to benefit 100 million poor families in the country by providing a cover of up to Rs 5 lakh (US$ 7,723.2) per family per year for secondary and tertiary care hospitalisation. It will help increase the per cent of population under health/ medical insurance. Thus now people would not have to spend on medical expenses from their savings and this increased expenditure on healthcare will benefit the pharmaceutical sector as well.
The government of India has announced a host of other measures to create a facilitating environment for the Indian pharmaceutical industry such as
- The allocation to the Ministry of Health and Family Welfare has increased by 11.5 per cent to Rs 52,800 crore (US$ 8.16 billion).
- The government has allocated Rs 1,200 core (US$ 185.36 million) towards the National Health Policy 2017 under which 150,000 health and wellness centers, will provide healthcare closer to homes of the people.
- Government of India is planning to create a digital platform to regulate and track the sale of quality drugs, which can be used by people living in the country as well as abroad.
The Indian Pharmaceutical market (IPM) accounts for approx.1.5% of the global pharmaceutical industry in value terms and 20% in the volume terms. Owing to robust historical growth, many MNC companies have active presence in the Indian pharmaceutical space.The IPM is highly fragmented with about 24,000 players (330 in the organized sector). The top tencompanies including domestic and MNC companies make up for more than athird of the market.The market is dominated majorly by branded generics, which constitutes nearly 70% of the overall market. Over the counter (OTC) medicines and patented drugs constitute 21% and 9% respectively.Besides the domestic market, Indian pharmaceutical companiesalso has a large chunk of their revenues coming from exports. Major companies have revenues coming in from the sale of intermediates, active pharmaceutical ingredients (APIs), and formulations in various global markets. These include developed markets like US, Europe and Japan and semi developed markets across the world. Some companies also derive revenues by providing custom research and manufacturing services to innovator companies. Biopharmaceuticals is also increasingly becoming an area of interest given the complexity in manufacture and limited competition.
Conclusion
The COVID 19 flare-up has additionally commenced the Indian pharmaceutical organizations an opportunity to transform into a supported trade place point for gathering drugs and intermediates.The ongoing COVID-19 pandemic has shown the Indian pharmaceutical sector in good light recently, while at the same time, it has significantly exposed the frailties of the sector. Currently, the Indian pharmaceutical sector is in the news for good reasons as it is able to export millions of doses of hydroxychloroquine, an antimalarial which, while not conclusively tested to be able to cure the coronavirus, has had some cases of making patients recover to countries like the United States and Brazil. However, the initial outbreak of the disease in China was a crisis for this very sector, as 70% of the active pharmaceutical ingredient (API) requirement was met by imports from China and as supplies for APIs, which is the principal raw material for drug manufacturers stopped. Due to this challenge that the sector faced during this crisis, various economic bodies in the country such as the NITI Aayog have mentioned that this needs to be addressed and a new policy must ensure that the manufacturing of APIs and pharmaceutical intermediates within the country should be promoted. While this is an obvious opportunity for pharmaceutical companies to backward integrate and start producing APIs and intermediates, it also provides companies in other sectors such as specialty chemicals to enter this sector. The domestic pharma industry is expected to grow at a 4-6 per cent in FY-2021 owing to Covid impact, though FY 2020-2023, CAGR is expected to be in the range of 8-11 per cent on the back of healthy demand from the domestic market given increasing spend on healthcare along with improving access. Along with demand growth in India, moderation in pricing pressure for US market, new launches and market share gains for existing products and consolidation benefits will drive growth for the industry over the medium term.